4WEB Clinical Publication | Article 1 of a 4-Part Series
09 . 20 . 22
Bone Mineralization and Spinal Fusion Evaluation of a Truss-Based lnterbody Fusion Device
Finite Element Analysis with Confirmatory Ovine In Vivo Outcomes
ABSTRACT
An ovine fusion study was conducted to evaluate the biologic response to different truss implant designs in order to determine the potential for Truss Implant Technology™ to enhance osteogenesis through optimized structural mechanics. Specifically, the goal was to determine comparative load-induced strain amplitudes, bone mineralization, and fusion outcomes associated with different design characteristics in a truss-based interbody fusion device.
BACKGROUND
Additive manufacturing technology has been employed to produce implants that actively participate in the fusion process. The truss device enables the transfer of compressive and tensile stresses via the struts. Mechanobiologic principles postulate that strut diameter can be regulated to allow different magnitudes of strain distribution within the struts which may affect fusion rates.
METHODS
Finite element analysis (FEA) was used to predict the strain distributions as a function of strut diameter under simulated physiologic loading conditions. An in vivo ovine lumbar spinal interbody fusion study compared fusion scores and bone histomorphometric variables for devices with differing strut diameters. Outcomes were compared at 3-, 6-, and 12-month timepoints.
RESULTS
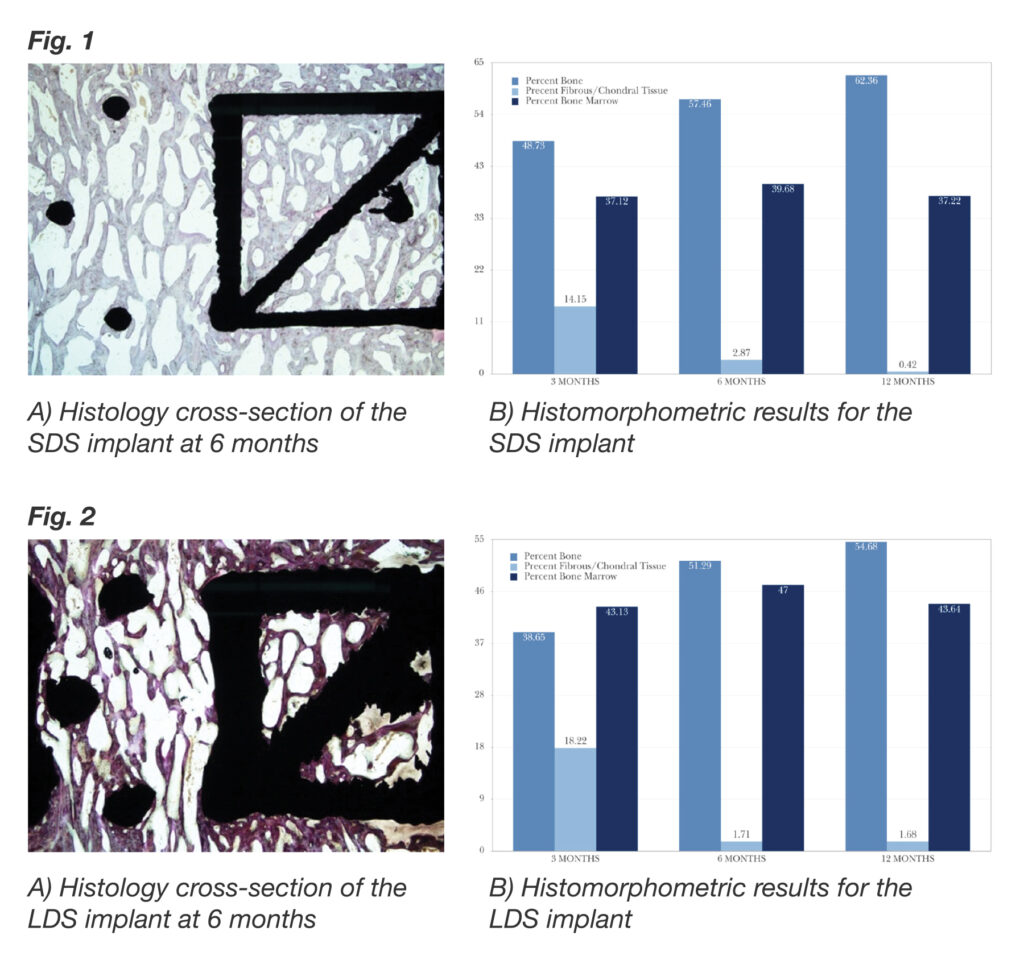
FEA showed an inverse association between strut diameter and peak strain amplitude. The results of the in vivo study showed that the small diameter strut (SDS) implant had a significantly higher mean fusion score than the large diameter strut (LDS) implant at the 3-month timepoint and remained significantly higher at each subsequent timepoint (P < 0.001 for all comparisons). Fusion rates were 95%, 100%, and 100% (SDS) and 72.7%, 86.4%, and 95.8% (LDS) at 3, 6, and 12 months, respectively. The SDS implant had greater mineralized bone tissue and less fibrous/chondral tissue than the LDS implant at each timepoint.
CONCLUSION
Both truss implants facilitated excellent fusion, and demonstrated overall results that are superior to those reported for conventional interbody devices used with autograft. The fact that the SDS implant performed better than the LDS implant with respect to greater percentage of bone formation for all timepoints, greater percentage of bone marrow ingrowth at all timepoints, and lower percentage of fibrous/chondral tissue and fibrous tissue thickness at most timepoints suggests that the SDS implant provides an improved mechanical environment for osseoincorporation.